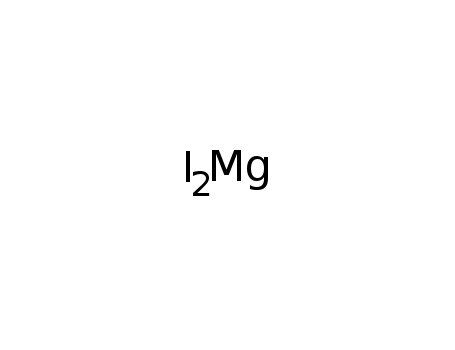- Molecular Formula: MgI2
- CasNo.: 10377-58-9
- Melting point: 637 °C (dec.)(lit.)
- Appearance: white crystalline solid
- ProductionCapacity:
- Purity:
- Packing:
-
Product Details:
High Purity Trustworthy Factory Supply 99.99% Magnesium iodide 10377-58-9 with Efficient Transportation
- Molecular Formula:I2Mg
- Molecular Weight:278.114
- Appearance/Colour:white crystalline solid
- Melting Point:637 °C (dec.)(lit.)
- PSA:0.00000
- Density:4.43 g/mL at 25 °C(lit.)
- LogP:1.77140
Magnesium iodide(Cas 10377-58-9) Usage
Use Description
Magnesium iodide, a specific chemical compound, serves varied purposes across different fields. In the field of organic synthesis, it acts as a Lewis acid catalyst, facilitating a range of reactions such as Friedel-Crafts reactions and Grignard reactions, enabling the formation of complex organic molecules for research and industrial applications. In the pharmaceutical industry, it may serve as a reactant or reagent in the synthesis of specific drug intermediates. Additionally, in the field of material science, magnesium iodide can be utilized as a precursor for the production of magnesium-based compounds with diverse properties. Its applications in organic synthesis, pharmaceuticals, and materials science underscore its importance in driving innovation, scientific research, and practical solutions across these distinct domains.
InChI:InChI=1/2HI.Mg/h2*1H;/q;;+2/p-2
10377-58-9 Relevant articles
Iodide-conducting polymer electrolytes based on poly-ethylene glycol and MgI2: Synthesis and structural characterization
Vittadello, Michele,Waxman, David I.,Sideris, Paul J.,Gan, Zhehong,Vezzù, Keti,Negro, Enrico,Safari, Ahmad,Greenbaum, Steve G.,Di Noto, Vito
, p. 112 - 122 (2011)
A major obstacle for a viable technologi...
Intramolecular Alkene Hydroamination with Hybrid Catalysts Consisting of a Metal Salt and a Neutral Organic Base
Fischer, Christian A.,Harder, Sjoerd,Langer, Jens,Nguyen, D. Thao,Penafiel, Johanne,R?sch, Andreas,Stegner, Philipp C.,Wiesinger, Michael
, p. 3387 - 3394 (2020)
Hybrid catalysts consisting of alkaline ...
A rechargeable non-aqueous Mg-O2 battery
Shiga, Tohru,Hase, Yoko,Kato, Yuichi,Inoue, Masae,Takechi, Kensuke
, p. 9152 - 9154 (2013)
We propose a catalytic cycle using the i...
Total Synthesis of Actinorhodin
Ninomiya, Mamiko,Ando, Yoshio,Kudo, Fumitaka,Ohmori, Ken,Suzuki, Keisuke
, p. 4264 - 4270 (2019)
The enantioselective total synthesis of ...
Proton NMR Characterization of the Ferryl Group in the Model Heme Complexes and Hemoproteins: Evidence for the FeIV=O Group in Ferryl Myoglobin and Compound II of Horseradish Peroxidase
Mar, Gerd N. La,Ropp, Jeffrey S. de,Latos-Grazynski, Lechoslaw,Balch, Alan L.,Johnson, R. B.,et al.
, p. 782 - 787 (1983)
The proton NMR spectra of model porphyri...
Crystal structures of hydrates of simple inorganic salts. I. Water-rich magnesium halide hydrates MgCl2·8H2O, MgCl 2·12H2O, MgBr2·6H2O, MgBr2·9H2O, MgI2·8H2O and MgI2·9H2O
Hennings, Erik,Schmidt, Horst,Voigt, Wolfgang
, p. 1292 - 1300 (2013)
The previously reported structures of th...
Redox active aluminium(iii) complexes convert CO2 into MgCO 3 or CaCO3 in a synthetic cycle using Mg or Ca metal
Myers, Thomas W.,Berben, Louise A.
, p. 4175 - 4177 (2013)
Redox-active Group 13 molecules possess ...
Total Synthesis of Gukulenin B via Sequential Tropolone Functionalizations
Nicolaou,Yu, Ruocheng,Lu, Zhaoyong,Alvarez, Fernando G.
supporting information, p. 5190 - 5196 (2022/03/27)
The synthesis of functionalized aromatic...
Efficient demethylation of aromatic methyl ethers with HCl in water
Bomon, Jeroen,Bal, Mathias,Achar, Tapas Kumar,Sergeyev, Sergey,Wu, Xian,Wambacq, Ben,Lemière, Filip,Sels, Bert F.,Maes, Bert U. W.
supporting information, p. 1995 - 2009 (2021/03/26)
A green, efficient and cheap demethylati...
Highly Active Superbulky Alkaline Earth Metal Amide Catalysts for Hydrogenation of Challenging Alkenes and Aromatic Rings
Eyselein, Jonathan,F?rber, Christian,Grams, Samuel,Harder, Sjoerd,Knüpfer, Christian,Langer, Jens,Martin, Johannes,Thum, Katharina,Wiesinger, Michael
supporting information, p. 9102 - 9112 (2020/03/30)
Two series of bulky alkaline earth (Ae) ...
10377-58-9 Process route
-
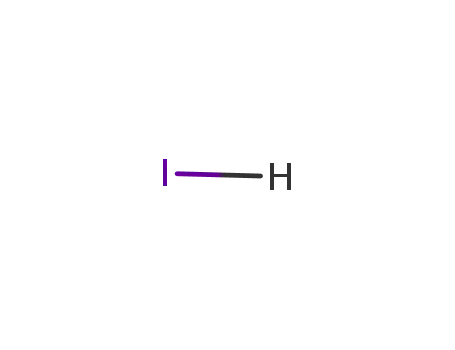
- 10034-85-2
hydrogen iodide

-
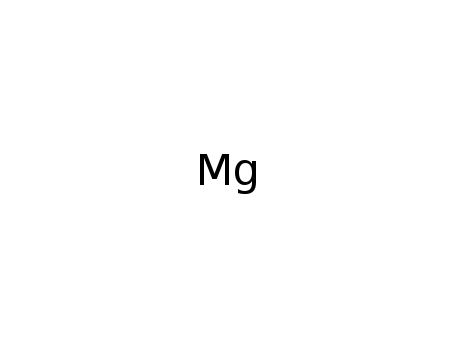
- 7439-95-4
magnesium

-
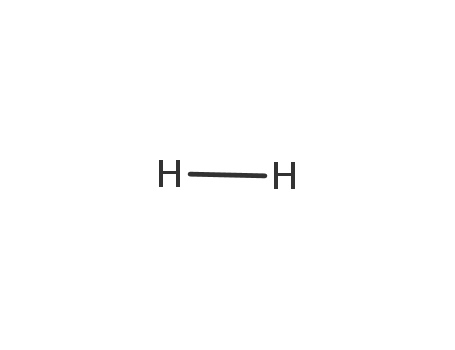
- 1333-74-0
hydrogen

-
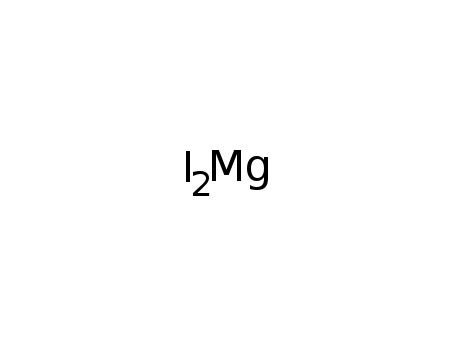
- 10377-58-9
magnesium iodide
ConditionsConditions Yield In benzene; reaction in presence of ether;;-
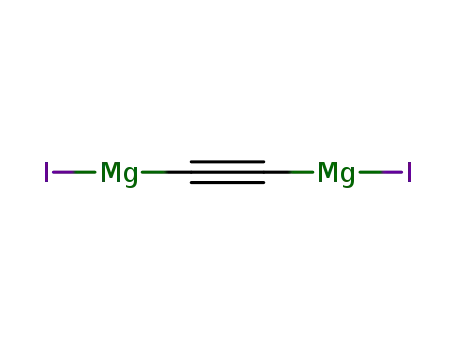
- 105238-67-3
Mg carbide iodide

-
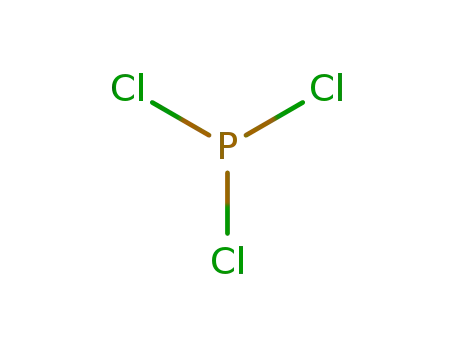
- 7719-12-2,52843-90-0
phosphorus trichloride

-
-
P2C6

-
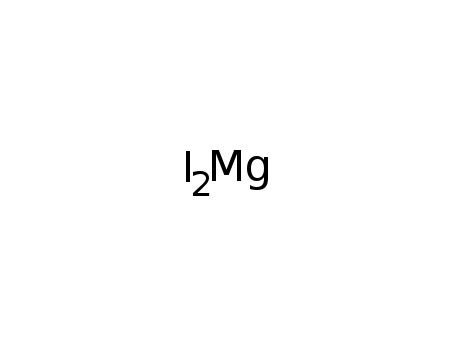
- 10377-58-9
magnesium iodide

-
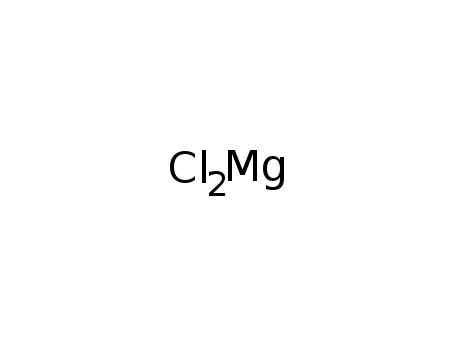
- 7786-30-3
magnesium chloride
ConditionsConditions Yield In diethyl ether; react. of a soln. of PCl3 in ether with IMgCCMgI; quantitative react.;;<99
<99In diethyl ether; reaction in ethereal soln. at ambient temperature;;In diethyl ether; react. of a soln. of PCl3 in ether with IMgCCMgI; quantitative react.;;<99
<99In diethyl ether; reaction in ethereal soln. at ambient temperature;;10377-58-9 Upstream products
-
7553-56-2
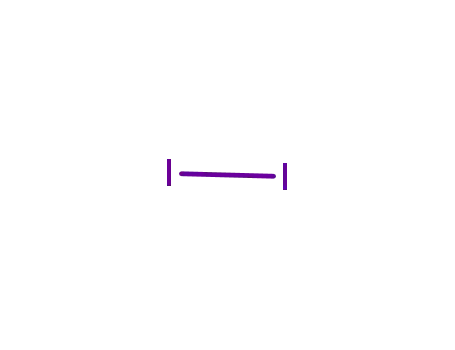
iodine
-
12035-89-1

strontium(II) oxide
-
7446-09-5
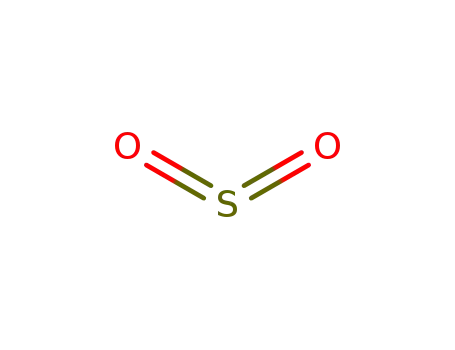
sulfur dioxide
-
7786-30-3
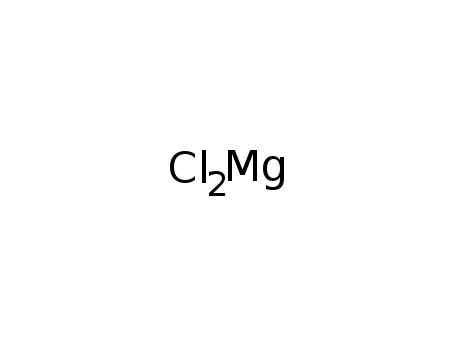
magnesium chloride
10377-58-9 Downstream products
-
7783-40-6
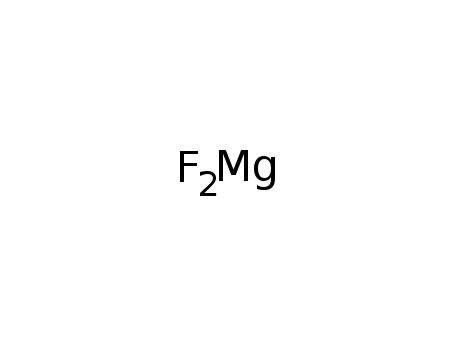
magnesium fluoride